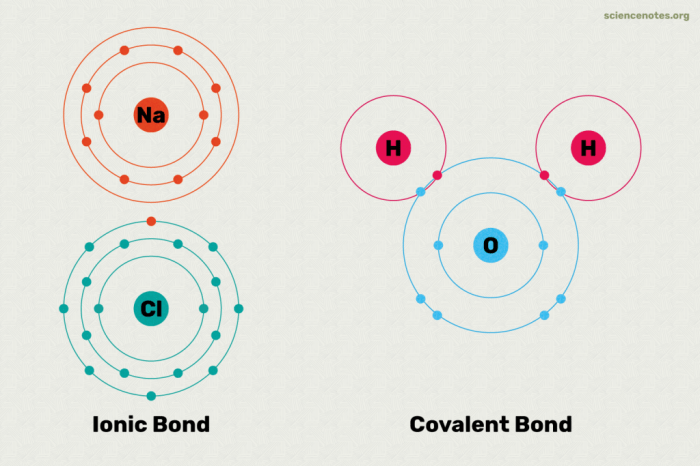
Dive into the world of ionic bonding with our comprehensive Ionic Bonding Gizmo Answer Key. Embark on a captivating journey to unravel the mysteries of ionic compounds, their properties, and their diverse applications in our daily lives.
Our detailed guide will illuminate the concepts and principles behind ionic bonding, empowering you to master the Gizmo simulation and gain a deeper understanding of this fundamental chemical concept.
Introduction to Ionic Bonding
Ionic bonding is a type of chemical bond formed between atoms of metals and non-metals. It is characterized by the transfer of electrons from the metal atom to the non-metal atom, resulting in the formation of positively charged ions (cations) and negatively charged ions (anions).Ionic
Looking for the ionic bonding gizmo answer key? If you’re also tackling the clc 222 module 4 exam, don’t forget to check out the clc 222 module 4 exam answers . Once you’ve got those covered, you’ll be well on your way to mastering ionic bonding.
compounds are typically formed when a metal atom loses one or more electrons to achieve a stable electron configuration, while a non-metal atom gains those electrons to achieve a stable electron configuration. The resulting electrostatic attraction between the oppositely charged ions holds the compound together.
Formation of Ionic Compounds
The formation of ionic compounds involves the following steps:
- Electron Transfer:A metal atom loses one or more electrons to achieve a stable electron configuration, becoming a positively charged cation.
- Electron Gain:A non-metal atom gains the electrons lost by the metal atom, becoming a negatively charged anion.
- Electrostatic Attraction:The oppositely charged ions are attracted to each other by electrostatic forces, forming an ionic bond.
Properties of Ionic Compounds
Ionic compounds are characterized by their distinct physical properties, which are influenced by the nature of their ionic bonding.
Ionic bonding involves the electrostatic attraction between positively charged cations and negatively charged anions. The strength of this attraction determines the properties of the compound.
Melting Point
Ionic compounds typically have high melting points due to the strong electrostatic forces between the ions. The melting point increases with the charge and size of the ions. Smaller ions with higher charges result in stronger electrostatic forces, requiring more energy to overcome and melt the compound.
Solubility
Ionic compounds are generally soluble in polar solvents, such as water. The polar solvent molecules interact with the ions, weakening the electrostatic forces and allowing the compound to dissolve. The solubility of an ionic compound depends on the size and charge of its ions, as well as the polarity of the solvent.
Electrical Conductivity
Ionic compounds conduct electricity in the molten state or when dissolved in a solvent. In the molten state, the ions are free to move and carry an electric current. In solution, the ions dissociate and become surrounded by solvent molecules, allowing them to conduct electricity.
Gizmo Answer Key
The Gizmo simulation on ionic bonding allows you to explore the process of forming ionic compounds and their properties.To complete the Gizmo simulation, follow these steps:
- Select two elements from the periodic table.
- Drag and drop the elements onto the reaction surface.
- Observe the formation of the ionic compound.
- Record the formula and properties of the ionic compound.
The Gizmo illustrates the following concepts and principles:* The formation of ionic compounds involves the transfer of electrons from one element to another.
- The resulting ions have opposite charges and are attracted to each other by electrostatic forces.
- Ionic compounds are typically solids with high melting and boiling points.
- Ionic compounds are soluble in water and conduct electricity in solution.
Step-by-Step Guide
- 1.
- *Select two elements from the periodic table. The elements should have different electronegativities, with one element being a metal and the other being a nonmetal.
- 2.
- *Drag and drop the elements onto the reaction surface. The elements will react to form an ionic compound.
- 3.
- *Observe the formation of the ionic compound. The ions will be arranged in a regular lattice structure.
- 4.
- *Record the formula and properties of the ionic compound. The formula will be determined by the charges of the ions. The properties will include the melting point, boiling point, and solubility in water.
Concepts and Principles
*
-*Formation of ionic compounds
Ionic compounds are formed when one element transfers electrons to another element. The resulting ions have opposite charges and are attracted to each other by electrostatic forces.
-*Properties of ionic compounds
Ionic compounds are typically solids with high melting and boiling points. They are also soluble in water and conduct electricity in solution.
Applications of Ionic Compounds
Ionic compounds are extensively utilized in various facets of daily life. Their unique properties, stemming from the strong electrostatic forces between positively charged cations and negatively charged anions, make them indispensable in numerous applications.
The functionality of ionic compounds in these applications is primarily attributed to their characteristic properties, including high melting and boiling points, good electrical conductivity, and the ability to form crystals with well-defined structures.
Electrolytes
Ionic compounds are crucial in the functioning of electrolytes, which are solutions that conduct electricity. When dissolved in water or other solvents, ionic compounds dissociate into their constituent ions, allowing the free movement of charged particles and facilitating the conduction of electrical current.
Electrolytes play a vital role in various applications, such as batteries, fuel cells, and electroplating.
Catalysts
Certain ionic compounds serve as catalysts, substances that accelerate chemical reactions without being consumed. The ionic nature of these compounds provides a favorable environment for specific reactions to occur, enhancing their efficiency and reducing the activation energy required.
Ionic catalysts are employed in a wide range of industrial processes, including the production of plastics, pharmaceuticals, and fertilizers.
Building Materials
Ionic compounds are essential components of many building materials, such as cement, concrete, and plaster. The strong ionic bonds between cations and anions contribute to the strength and durability of these materials, making them suitable for construction purposes.
The use of ionic compounds in building materials ensures structural integrity, longevity, and resistance to environmental factors.
Medicine
Ionic compounds find applications in the medical field, particularly in the development of drugs and treatments. The precise control over the composition and properties of ionic compounds allows for the creation of tailored pharmaceuticals with specific therapeutic effects.
Ionic compounds are utilized in antibiotics, antacids, and electrolyte solutions, among other medical applications.
Food Additives
Ionic compounds are commonly used as food additives to enhance flavor, texture, and preservation. They play a crucial role in maintaining the stability and quality of processed foods.
For instance, sodium chloride (table salt) is an essential seasoning agent, while calcium chloride is used as a firming agent in canned vegetables.
Examples of Ionic Compounds: Ionic Bonding Gizmo Answer Key
Ionic compounds are formed when a metal loses one or more electrons to a nonmetal. The resulting ions are attracted to each other by electrostatic forces, forming a crystal lattice. Ionic compounds are typically hard, brittle, and have high melting points.
Some common ionic compounds include:
Table of Common Ionic Compounds
| Compound | Formula | Uses |
|---|---|---|
| Sodium chloride (table salt) | NaCl | Seasoning food, preserving food |
| Potassium chloride | KCl | Fertilizer, substitute for salt |
| Calcium carbonate (limestone) | CaCO3 | Construction materials, antacids |
| Magnesium oxide (magnesia) | MgO | Antacids, fertilizer |
| Sodium bicarbonate (baking soda) | NaHCO3 | Baking, cleaning |
| Potassium nitrate (saltpeter) | KNO3 | Fertilizer, gunpowder |
| Copper sulfate (blue vitriol) | CuSO4 | Fungicide, algicide |
| Iron(II) sulfate (ferrous sulfate) | FeSO4 | Fertilizer, food additive |
| Iron(III) chloride (ferric chloride) | FeCl3 | Coagulant, etching agent |
| Aluminum chloride | AlCl3 | Catalyst, deodorant |
Limitations of Ionic Bonding
Ionic bonding has several limitations that affect its practical applications. These limitations include sensitivity to water and high melting points.
Sensitivity to Water, Ionic bonding gizmo answer key
Ionic compounds are typically soluble in water. When an ionic compound dissolves in water, the water molecules surround the ions and separate them from each other. This process is called dissociation. Dissociation can weaken the ionic bond and lead to the formation of ions in solution.
High Melting Points
Ionic compounds typically have high melting points. This is because the ions in an ionic compound are strongly attracted to each other, making it difficult to break the ionic bonds and melt the compound.
The limitations of ionic bonding can affect the practical applications of ionic compounds. For example, ionic compounds that are soluble in water cannot be used in applications where they will be exposed to water. Similarly, ionic compounds with high melting points cannot be used in applications where they will be subjected to high temperatures.
General Inquiries
What is ionic bonding?
Ionic bonding is a chemical bond formed between two oppositely charged ions, typically a positively charged metal ion and a negatively charged non-metal ion.
How do I use the Ionic Bonding Gizmo?
Follow the step-by-step instructions provided in our answer key to navigate the Gizmo simulation effectively and explore the concepts of ionic bonding.
What are some examples of ionic compounds?
Common ionic compounds include sodium chloride (table salt), potassium chloride, and calcium oxide, each with unique properties and applications.