Predict the products of the following Diels-Alder reactions. The Diels-Alder reaction is a powerful tool for the synthesis of cyclic compounds, and it is one of the most important reactions in organic chemistry. The reaction involves the addition of a conjugated diene to a dienophile, resulting in the formation of a cyclic product.
The regioselectivity and stereoselectivity of the reaction can be controlled by a variety of factors, including the nature of the diene and dienophile, the reaction conditions, and the presence of catalysts.
In this article, we will discuss the mechanism of the Diels-Alder reaction, the factors that affect its regioselectivity and stereoselectivity, and the applications of the reaction in organic synthesis. We will also provide examples of Diels-Alder reactions with different dienes and dienophiles.
1. Introduction
The Diels-Alder reaction is a powerful cycloaddition reaction in organic chemistry that involves the reaction of a conjugated diene with a dienophile to form a cyclic product. It is one of the most important reactions in organic synthesis due to its ability to form complex and functionalized molecules.
2. Mechanism of the Diels-Alder Reaction
Stepwise Mechanism
The Diels-Alder reaction proceeds through a concerted pericyclic mechanism. The first step involves the formation of a transition state, where the diene and dienophile interact through a [4+2] cycloaddition. In the second step, the transition state collapses to form the cyclic product.
Role of the Diene and Dienophile
The diene must be conjugated to allow for the formation of the aromatic ring in the product. The dienophile can be a variety of compounds, including alkenes, alkynes, and carbonyl compounds.
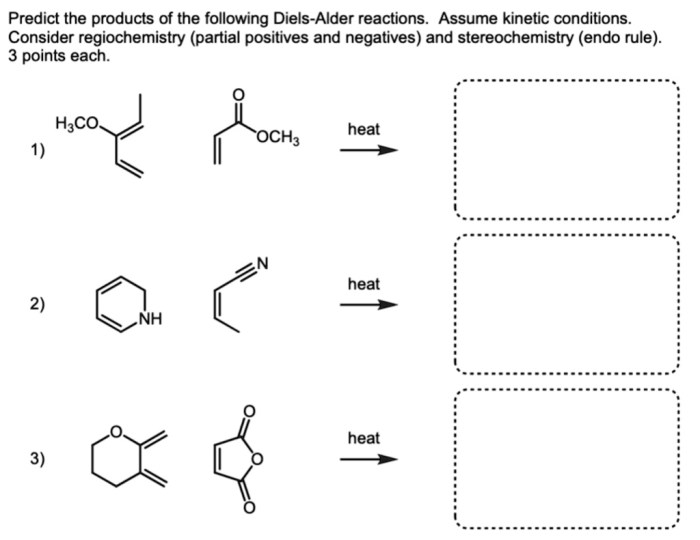
3. Predicting the Products of Diels-Alder Reactions
Factors Affecting Regioselectivity and Stereoselectivity
The regioselectivity of the Diels-Alder reaction is determined by the relative reactivity of the diene and dienophile. The stereoselectivity is determined by the orientation of the diene and dienophile in the transition state.
Guidelines for Predicting the Major Product
- The diene will react with the dienophile in a way that maximizes the number of substituted carbons in the product.
- The dienophile will add to the diene in a way that minimizes steric hindrance.
- The dienophile will add to the diene in a way that creates the most stable product.
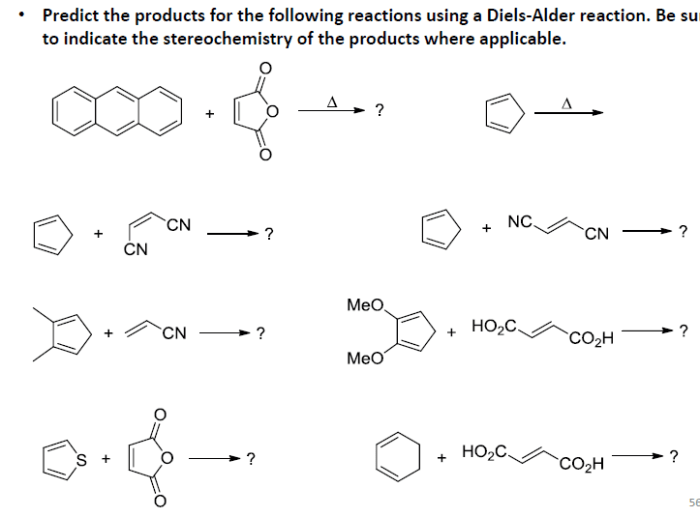
4. Examples of Diels-Alder Reactions
Reaction with Butadiene and Ethylene
The reaction of butadiene with ethylene yields cyclohexene as the major product. The reaction is regioselective for the formation of the substituted cyclohexene.
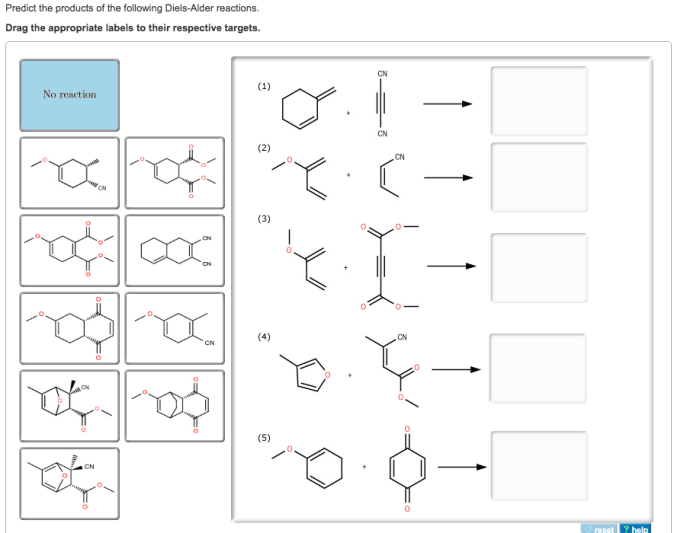
Reaction with Cyclopentadiene and Maleic Anhydride, Predict the products of the following diels-alder reactions
The reaction of cyclopentadiene with maleic anhydride yields a Diels-Alder adduct that can undergo a subsequent cyclization reaction to form a bicyclic product.
5. Applications of Diels-Alder Reactions: Predict The Products Of The Following Diels-alder Reactions
The Diels-Alder reaction is a versatile tool for the synthesis of a wide range of organic compounds. It is used in the synthesis of natural products, pharmaceuticals, and polymers.
One important application of the Diels-Alder reaction is in the synthesis of steroids. Steroids are a class of organic compounds that have a characteristic four-ring structure. The Diels-Alder reaction can be used to form the steroid nucleus.
General Inquiries
What is the Diels-Alder reaction?
The Diels-Alder reaction is a cycloaddition reaction between a conjugated diene and a dienophile, resulting in the formation of a cyclic product.
What are the factors that affect the regioselectivity and stereoselectivity of the Diels-Alder reaction?
The regioselectivity and stereoselectivity of the Diels-Alder reaction can be controlled by a variety of factors, including the nature of the diene and dienophile, the reaction conditions, and the presence of catalysts.
What are the applications of the Diels-Alder reaction in organic synthesis?
The Diels-Alder reaction is widely used in organic chemistry for the synthesis of a variety of cyclic compounds, including natural products and pharmaceuticals.