Lithium sulfide and copper II nitrate are two important inorganic compounds with distinct chemical properties and applications. Lithium sulfide, a white solid, is primarily used in batteries and glass production, while copper II nitrate, a blue crystalline solid, finds applications in fertilizers, pigments, and wood preservatives.
This comprehensive overview delves into the synthesis, reactivity, and environmental impact of these compounds, providing a thorough understanding of their significance in various industrial and scientific fields.
1. Lithium Sulfide and Copper II Nitrate
An Overview
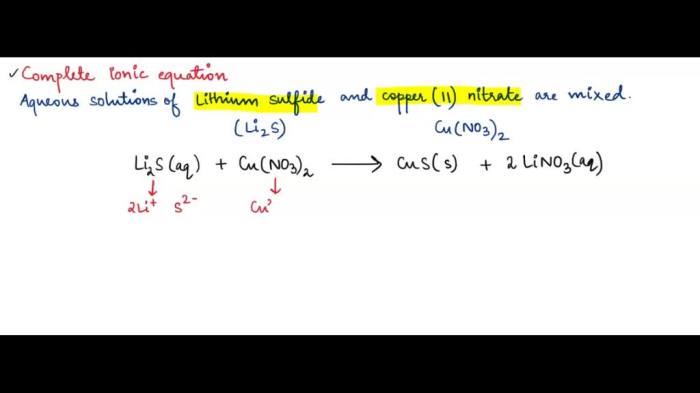
Lithium sulfide (Li 2S) and copper II nitrate (Cu(NO 3) 2) are inorganic compounds with distinct chemical properties and applications. Lithium sulfide is a colorless, crystalline solid, while copper II nitrate is a blue, deliquescent solid.
Lithium sulfide is highly reactive with water, forming lithium hydroxide (LiOH) and hydrogen sulfide (H 2S) gas. It is also soluble in organic solvents such as ethanol and dimethylformamide. Copper II nitrate is soluble in water and decomposes upon heating, releasing nitrogen dioxide (NO 2) and oxygen (O 2).
Lithium sulfide finds applications in the production of lithium batteries, glass, and lubricants. Copper II nitrate is used as a fertilizer, pigment, and wood preservative.
2. Synthesis of Lithium Sulfide and Copper II Nitrate
Synthesis of Lithium Sulfide
Lithium sulfide can be synthesized by reacting lithium metal with sulfur powder in an inert atmosphere.
- 2Li + S → Li 2S
The reaction is exothermic, and the product is a colorless, crystalline solid.
Synthesis of Copper II Nitrate
Copper II nitrate can be synthesized by reacting copper metal with nitric acid.
- Cu + 4HNO 3→ Cu(NO 3) 2+ 2NO 2+ 2H 2O
The reaction is carried out in a fume hood due to the release of nitrogen dioxide gas. The product is a blue, deliquescent solid.
3. Reactivity and Reactions of Lithium Sulfide and Copper II Nitrate
Reactivity of Lithium Sulfide
Lithium sulfide is highly reactive with water, forming lithium hydroxide (LiOH) and hydrogen sulfide (H 2S) gas.
- Li 2S + 2H 2O → 2LiOH + H 2S
Lithium sulfide is also reactive with acids, forming lithium salts and hydrogen sulfide gas.
- Li 2S + 2HCl → 2LiCl + H 2S
Reactions of Copper II Nitrate
Copper II nitrate is reactive with bases, forming copper(II) hydroxide (Cu(OH) 2) and nitrate ions (NO 3–).
- Cu(NO 3) 2+ 2NaOH → Cu(OH) 2+ 2NaNO 3
Copper II nitrate is also reactive with reducing agents, forming copper(I) compounds and nitrogen oxides.
- 2Cu(NO 3) 2+ SnCl 2→ 2CuNO 3+ SnCl 4+ 2NO
4. Applications of Lithium Sulfide and Copper II Nitrate

Applications of Lithium Sulfide, Lithium sulfide and copper ii nitrate
Lithium sulfide finds applications in the production of lithium batteries, glass, and lubricants.
- Lithium batteries:Lithium sulfide is used as a cathode material in lithium-sulfur batteries, which are lightweight and have a high energy density.
- Glass:Lithium sulfide is added to glass to improve its strength and durability.
- Lubricants:Lithium sulfide is used as an additive in lubricants to reduce friction and wear.
Applications of Copper II Nitrate
Copper II nitrate is used as a fertilizer, pigment, and wood preservative.
- Fertilizer:Copper II nitrate is a source of copper for plants, which is essential for photosynthesis and growth.
- Pigment:Copper II nitrate is used to produce green and blue pigments for paints and ceramics.
- Wood preservative:Copper II nitrate is used to treat wood to prevent rot and decay.
FAQs
What is the chemical formula of lithium sulfide?
Li2S
What is the color of copper II nitrate?
Blue
Are lithium sulfide and copper II nitrate toxic?
Yes, both compounds can be toxic if ingested or inhaled.